Abstract
Background: Recently, therapeutics targeting B-cell Maturation Antigen (BCMA), including CAR T cells, bispecific antibodies (bsAbs) and antibody-drug conjugates (ADCs), have shown promise in improving outcomes in RRMM patients (pts). However, targeting BCMA can impair natural humoral immunity and increase infection risk. It remains unknown if BCMA-targeted therapies predispose pts to infection at higher frequency or with different characteristics than non-BCMA-targeted therapies in this RRMM population, or if the frequency and characteristics of infections differ amongst the various BCMA-targeted modalities.
Methods: We conducted a single-center retrospective study of RRMM pts who started BCMA-targeted therapies (CAR T, bsAb, or ADC) or a selinexor-based treatment between Nov. 2015 and May 2021. We extracted data on patient demographics, MM characteristics, prior lines of therapy, neutrophil and lymphocyte counts, and response to therapy, as well as frequency, type, and severity of infections, quantitative immunoglobulins, and use of intravenous immunoglobulin (IVIG). Pts who had received serial BCMA-targeted therapies, or both a BCMA-targeted therapy and selinexor were excluded from analysis.
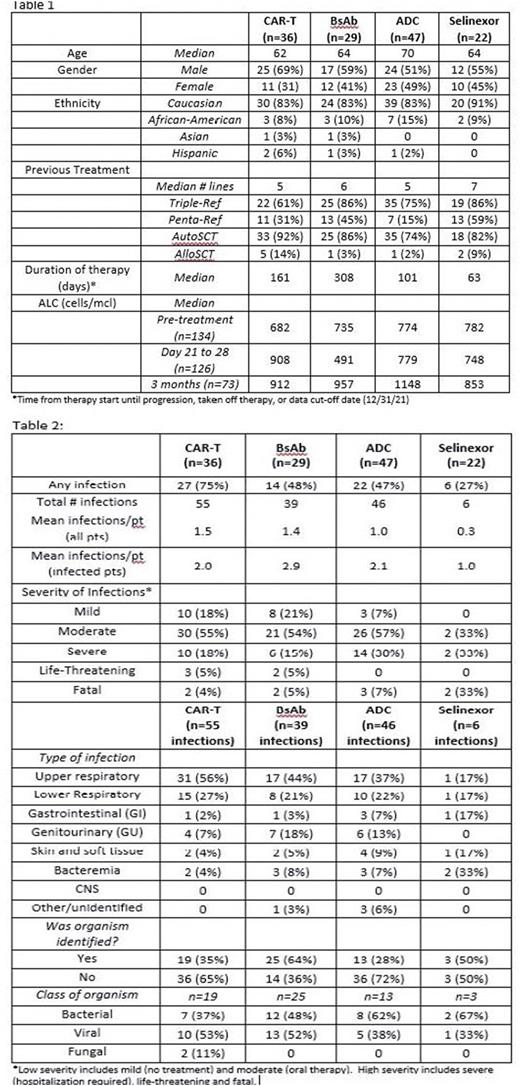
Results: There were 134 pts included in the analysis: 112 who received a BCMA-targeted treatment (36 CAR T, 29 bsAb, and 47 ADC) and 22 who received selinexor-based treatment. Overall pt characteristics, including age, median lines of therapy, and %triple- and penta-refractory, were similar between the anti-BCMA therapy and selinexor groups, as well as amongst pts receiving the different anti-BCMA modalities. However median duration of therapy differed between groups, longest in the bsAb group and shortest for selinexor (Table 1). Of pts receiving BCMA-targeted treatment, 63 (56%) experienced at least 1 infectious complication (75% of CAR T, 48% of bsAb, and 47% of ADC pts), compared to 6 pts (27%) in the selinexor group. These 69 pts had a total of 146 infections (mean of 2.0, 2.9, 2.1, and 1.0 infections per infected pt in the CAR T, bsAb, ADC, and selinexor groups, respectively) (Table 2). Of the infections in the BCMA-treated pts, 70% were considered low-severity (no treatment or oral treatment alone) and 30% were high-severity (hospitalization, life-threatening, or fatal), with similar frequency of high-severity infections amongst pts getting CAR T, bsAb or ADC. Four of 6 infections (67%) in the selinexor group were high-severity. Most (66%) of the low-severity were upper respiratory tract infections, while most high-severity were lower respiratory tract infections (54%) or bacteremia (22%). Low-severity infections were most commonly viral, while high-severity infections were more likely bacterial. Fungal infections were rare (n=2, both in CAR-T patients). Median time to onset of high-severity infections was 59 days in the CAR T cohort compared to 278 days and 124 days in the bsAb and ADC groups, respectively, and 205 days in the selinexor group. Only 12 of the 146 infections (8%) occurred while pts were neutropenic. Median absolute lymphocyte counts (ALC) were low in all groups pre-treatment; amongst pts still on therapy at 3 months, median ALC had increased in all 3 BCMA-treated groups (Table 1). Regarding hypogammaglobulinemia we focused on pts with non-IgG myeloma to limit confounding effects of the monoclonal IgG protein. Amongst non-IgG pts receiving BCMA-targeted treatments (n=50), 70% had IgG<400 pre-treatment, which increased to 87% by 3 months. For non-IgG selinexor pts (n=9), these numbers were 67% and 71%, respectively. At least 1 dose of IVIG was given in 31% of BCMA-treated pts (22% of CAR T, 59% of bsAb, and 21% of ADC).
Conclusions: RRMM pts treated with BCMA-targeted therapies are at high risk of developing an infectious complication, regardless of treatment modality, with many patients having multiple infections, and up to 30% developing high-severity infections. Infection frequency in this study appeared higher for anti-BCMA-treated pts than for selinexor-treated patients, though this finding is confounded by the small numbers and shorter duration of therapy in the selinexor group. Further analyses of the impact of response, ALC, immunoglobulin levels, and IVIG use on infection risk and severity are ongoing. These data demonstrate a need for optimizing strategies to prevent and treat infectious complications in RRMM pts receiving BCMA-targeted therapies.
Disclosures
Garfall:Novartis, Janssen, Tmunity, CRISPR Therapeutics: Research Funding; Janssen, GSK, Amgen, Legend: Consultancy. Vogl:Oncopeptides: Consultancy; Karyopharm: Consultancy; CSL/Behring: Consultancy; Sanofi/Genzyme: Consultancy; GSK: Consultancy; Takeda: Consultancy, Research Funding; Active Biotech: Research Funding. Stadtmauer:BMS, Celgene, Abbvie, Sorrento: Research Funding. Cohen:Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Ichnos, Janssen Oncology, Oncopeptides, Pfizer, Seattle Genetics, Genentech/Roche, AstraZeneca, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline and Novartis: Research Funding; Novartis: Patents & Royalties: CAR T-cells and biomarkers of cytokine-release syndrome.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal